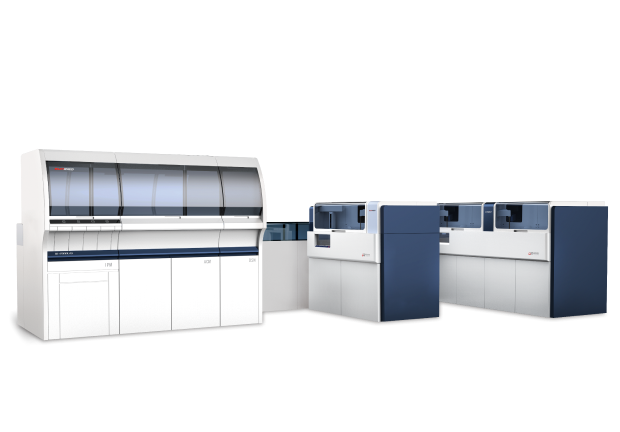
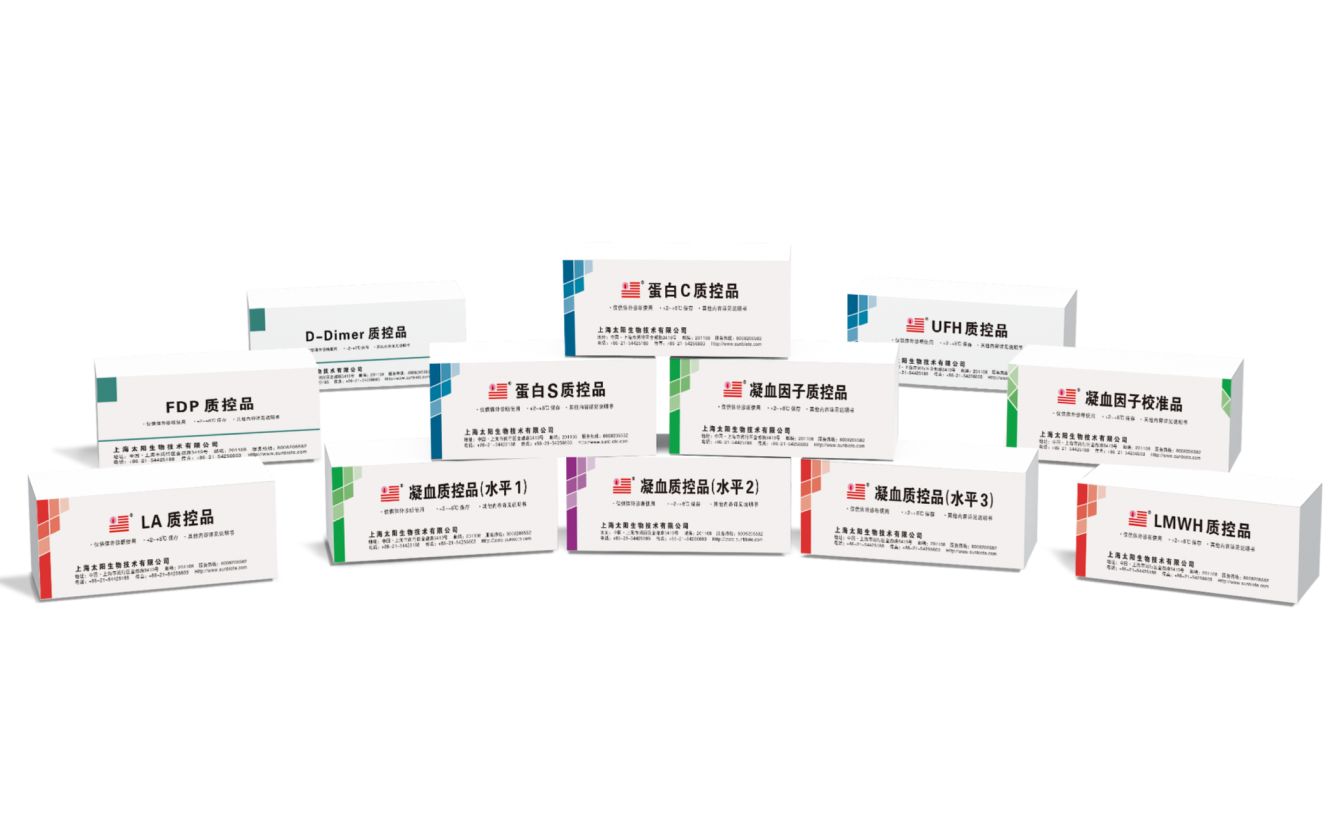
Shanghai Sun Biotech Co., Ltd., founded in 2001, is a global mainstream supplier of thrombosis and hemostasis total solutions, and continues to focus on the research, development, production and sales of diagnostic reagents for thrombosis and hemostasis, medical equipment and medical consumables, etc.
We have leading R&D and production capacity in the field of thrombosis and hemostasis, and have built the largest R&D and production base for diagnostic reagents, medical equipment and medical consumables in China. We have been accredited as a CNAS Medical Reference Laboratory by the China National Accreditation Service for Conformity Assessment (CNAS) and strictly adhere to the quality management system. Our certifications include ISO9001 and ISO13485 dual international quality system certifications, and CE product certification. Leveraging our outstanding product performance, professional customer service and comprehensive marketing network, we have established close partnerships with global medical institutions.
We will continue to push boundaries of scientific and technological innovation, explore the cutting-edge applications, and provide accurate, efficient and accessible diagnostic solutions for global medical institutions and patients through our high-quality thrombosis and hemostasis diagnostic products, leading the development of diagnostic business and shaping the future of human health.

-
VISION
To be the global leader in thrombosis and hemostasis diagnostics
-
MISSION
INVENT for LIFE
-
VALUES
Centered on Customers, Driven by Strivers

The Future, More Than Exploration
Unlimited Innovation, Breaking through the Unknown
-
-
2024


-
5 analytes, 3 levels coagulation control certified (PT/APTT/TT/FIB/AT-III)
-
-
2024


-
Shanghai Brand Cultivation Benchmarking Enterprise
-
-
2023


-
World's first ultra-fast UR8000 automated coagulation analyzer launched
-
-
2023


-
New-generation UR6000 constant-throughput automated coagulation analyzer launched
-
-
2023


-
Recognized as one of the first"Shanghai Intelligent Factories"
-
-
2022


-
"Shanghai Enterprise Technology Center" was officially inaugurated
-
UP5500 - The first constant-throughput automated coagulation analyzer was launched
-
-
2021


-
Recognized as an SRDI "Little Giant" enterprise by the Ministry of Industry and Information Technology
-
-
2021


-
UP3000 - The first automated coagulation analyzer officially launched
-
-
2020


-
Selected as a member enterprise of China Medical Device Industry Association
-
-
2019


-
AT-III reagent - Shanghai Top 100 High-tech Achievement Transformation Projects
-
-
2019


-
Awarded the title of Shanghai Science and Technology "Little Giant" Enterprise
-
-
2018

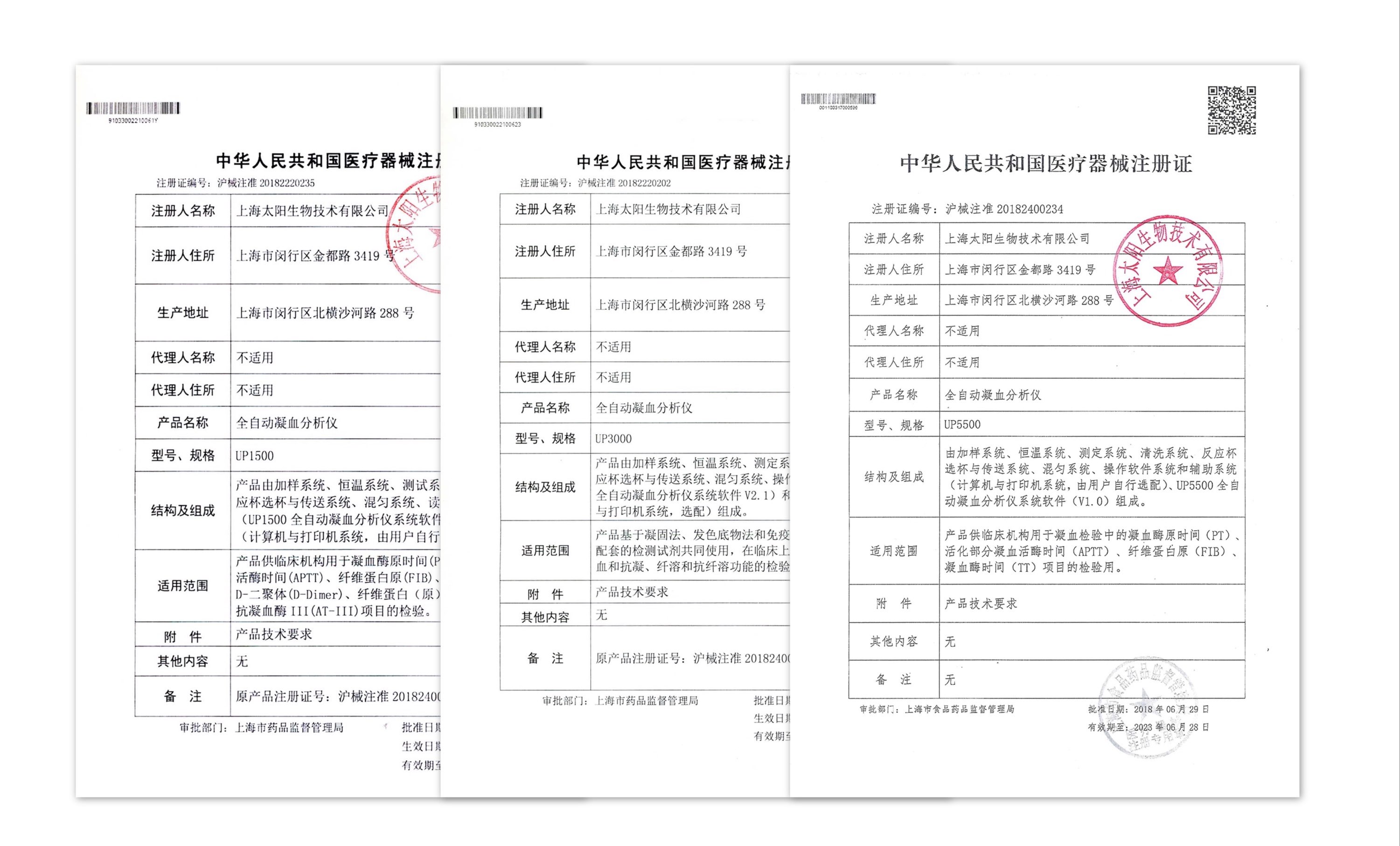
-
UP1500, UP3000, UP5000, UP5500 fully automatic coagulation analyzer registration certificate approved
-
-
2017


-
PT, FIB, D-Dimer reagents were honored as "Shanghai Outstanding Medical Device Products”
-
-
2017


-
Awarded as an Outstanding Science and Technology Enterprise at the First International Science and Technology Parks (Shanghai) Expo
-
-
2016


-
Established an R&D and production base for medical equipment and consumables
-
-
2015


-
Undertook the National Development and Reform Commission's pilot project for regional cluster development of strategic emerging industries in biomedical engineering
-
-
2014


-
Undertook Shanghai's Strategic Emerging Industry Project
-
-
2014


-
The D-Dimer reagent was recognized as one of Shanghai's top ten independent innovations in high-tech achievement transformation project
-
-
2013


-
Awarded as"Shanghai Science and Technology Little Giant (Cultivation) Enterprise"
-
-
2012


-
FDP and D-Dimer reagents were recognized as part of "Shanghai High-tech Achievement Transformation Project"
-
-
2012


-
Awarded as"Shanghai Innovative Enterprise"
-
The D-Dimer reagent was approved as a project funded by the National Technology Innovation Fund for Small and Medium-sized Enterprises project
-
-
2010


-
First in China to launch D-Dimer, FDP and ATIII reagents for fully automated coagulation analyzers
-
-
2009


-
Established a GMP-compliant diagnostic reagent R&D and production base spanning over 10,000 square meters
-
-
2007


-
Launched machine-specific liquid reagents for different brands of coagulation analyzers
-
-
2006


-
Established a R&D and production base for consumables
-
-
2005


-
Obtained national high-tech enterprise certification for the first time
-
-
2004

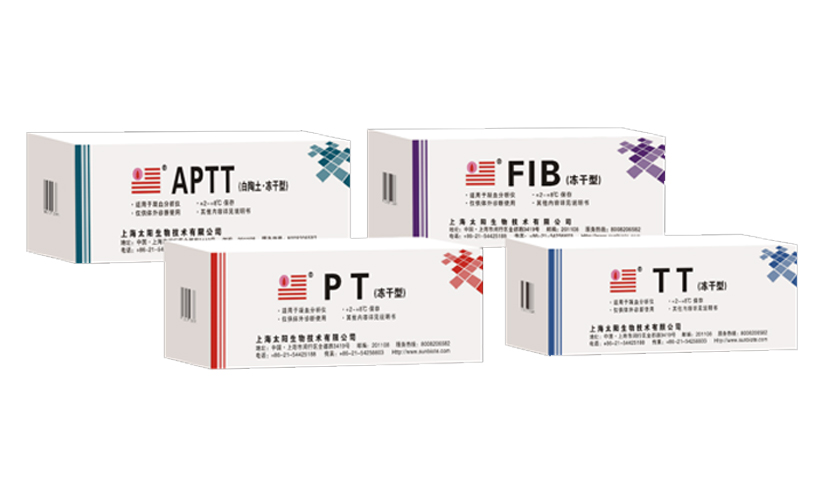
-
Launched machine-specific lyophilized reagents for different brands of coagulation analyzers
-
-
2001


-
Shanghai Sun Biotech Co., Ltd. was founded
-
Obtained the Medical Device Manufacturing License
-
Load more