Through 4 years production base construction and R&D tackling, on the 19th November 2018, the “IVD full automatic coagulation analyzer industrial project” as a key project of shanghai strategic emerging industry undertook by our company passed the acceptance successfully by the evaluation of Shanghai Science and Technology Commission, Shanghai Development and Reform Commission and industry experts.
After verified project implementation condition on the spot, listened to the project report, reviewed files of project acceptance, and questioned on the spot, review panel gave fully affirmation finally. They agreed that the overall objectives of this project has reached the expected targets, science technology tackling goals, intellectual property goals, industrialization target and other detailed acceptance standards, and agreed to pass the acceptance.

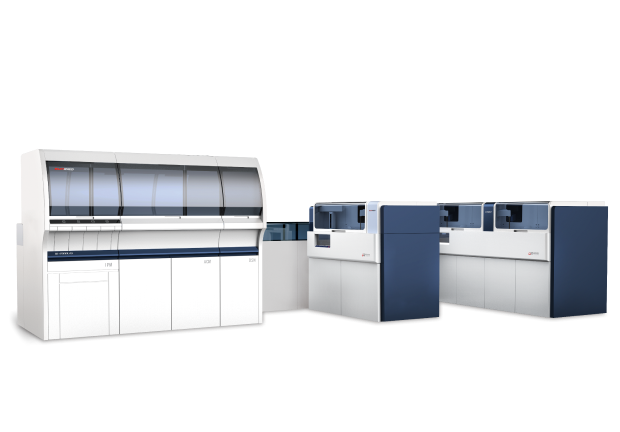
Totally 89.4812 million RMB was invested in the “IVD Full Automatic Coagulation Analyzer Industrilization Project “ , including 12.6 million RMB that funded by Shanghai Development and Reform Commission, Science and Technology Commission, Minhang Science and Technology Commission. The project completed the construction of the industrialization base of the automatic coagulation analyzer with a total construction area of more than 20,000 square meters, and successfully passed the completion acceptance. Our company has successfully developed automatic coagulation analyzer UP1500, UP3000,UP5000.These three analyzers had passed inspection of National Food and Drug Administration and Shanghai Medical Equipment Quality Control Supervision and Inspection Center, also achieved medical equipment registration licenses. Sunbiohas completed the construction of production line, equipped with advanced R&D, production and inspection equipment, had mass production capability.
The successful implementation of IVD full automatic coagulation analyzer is beneficial to enhance the R&D strength of our company, improving the independent innovation ability of enterprises, improving the technological level of domestic medical equipment products, enhancing the market competiveness of domestic medical equipment, and promoting the development of domestic medical equipment industry.